Are you curious about the crucial details behind your favorite cosmetic products? Ever wondered how regulatory bodies ensure the safety of these products on the market? Look no further – welcome to the comprehensive guide on Cosmetic Product Information Files (PIFs). In this article, we’ll delve into the depths of PIFs, uncovering their importance, components, creation process, and much more. So, let’s embark on a journey to demystify the world of cosmetic product regulation!

Table of Contents
Introduction to Cosmetic Product Information File (PIF)
When you hold a cosmetic product in your hands, you’re actually holding a result of extensive research, testing, and documentation. This is where the Cosmetic Product Information File, or PIF, takes the stage. A PIF is a comprehensive dossier that contains a treasure trove of information about a cosmetic product – from its formulation to its packaging, all the way to safety assessments.
Why Cosmetic Product Information Files Are Vital
PIFs play a pivotal role in ensuring consumer safety and product efficacy. Regulatory authorities worldwide require manufacturers to maintain PIFs for every cosmetic product they place on the market. These files serve as a reliable source of information for regulators to assess the product’s compliance with safety and quality standards.
Key Components of a Cosmetic Product Information File
A well-structured PIF comprises several key components, including:
- Product Description and Intended Use
- Formulation Details and Ingredients
- Manufacturing Process and Quality Control
- Packaging Information
- Safety Data, Assessments, and Test Results
- Exposure and Use Information
Crafting a Comprehensive Cosmetic Product Information File
Creating a robust PIF involves meticulous gathering of data, collaboration among different departments, and adherence to regulatory guidelines. Manufacturers must ensure that all information is accurate, up-to-date, and easily accessible.
Regulatory Guidelines and Compliance
Regulatory bodies, such as the FDA and the European Commission, provide specific guidelines for creating PIFs. Adhering to these guidelines ensures that products meet the necessary safety and labeling requirements before entering the market.
Safety Assessment and Risk Evaluation
Safety assessments are a cornerstone of PIFs. These evaluations involve identifying potential risks associated with a product’s ingredients and usage, ensuring that the product is safe for consumers.
PIF Maintenance and Updates
PIFs aren’t static documents; they require consistent maintenance and updates as new information becomes available. Manufacturers must monitor the safety and efficacy of their products and reflect any changes in the PIF accordingly.
PIF vs. Product Labeling: What’s the Difference?
While both PIFs and product labels provide essential information to consumers, PIFs are more extensive and detailed. They contain in-depth data that goes beyond what’s available on the product packaging.
The Future of PIFs: Technological Advancements
As technology advances, PIF creation and management are becoming more streamlined. Digital tools and platforms are emerging to facilitate efficient PIF generation, storage, and updates.
Industry Insights: Expert Interviews
We’ve interviewed industry experts to gain insights into the world of PIFs, their significance, and the challenges faced by manufacturers in creating and maintaining them.
Common Misconceptions About Cosmetic Product Information File
There are several misconceptions surrounding PIFs. We debunk these myths and provide accurate information about their purpose and usage.
Addressing Consumer Concerns
Consumers often have questions about the products they use. Manufacturers can use PIFs to address common concerns and provide transparent information.
Behind the Scenes: How PIFs Impact Purchasing Decisions
Unbeknownst to many consumers, PIFs influence their purchasing decisions. Understanding PIFs empowers consumers to make informed choices.
Step-by-Step Guide: Developing PIFs Effectively
As a manufacturer, it is crucial to create a comprehensive Cosmetic Product Information File (PIF) to ensure the safety and compliance of your cosmetic products. Our detailed step-by-step guide will assist you in developing effective PIFs that are well-documented. Follow these steps to achieve successful results.
Step 1: Gather Essential Information For Your Cosmetic Product Information File
Start by collecting all the necessary information about your cosmetic product. This includes its formulation, ingredients, packaging details, intended use, and any safety assessments conducted etc.
What is included in a Cosmetic Product Information File (PIF)?
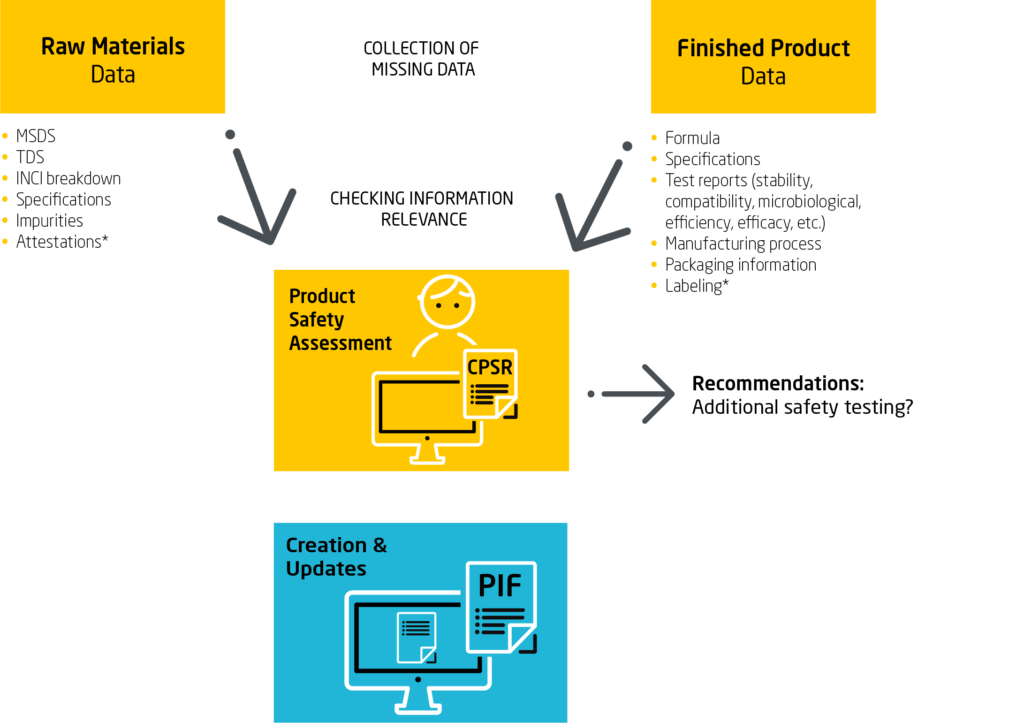
Where Do I Find the Right Data For My Cosmetic Product Information File?
Some of the data comes from:
- Product manufacturers
Other data comes from :
- An independent laboratories,
- A duly qualified safety assessor with degrees recognized .
which Documents and Information Must Be Part Of the Cosmetic Product Information File
| 1. A description of the cosmetic product which enables the product information file to be clearly attributed to the cosmetic product |
| 2. The CPSR part A and B |
| 3. The annexes of the CPSR |
| 4. Formula in raw materials |
| 5. Specification sheet and/or certificate of analysis of the finished product |
| 6. Documentation on each raw material (MSDS, CofA, IFRA and allergen report if applicable) |
| 7. Stability study |
| 8. Microbiological information (challenge tests – microbiological analysis) |
| 9. Information about the packaging material (material description, impurities statement, food contact statement,) |
| 10. Toxicological evaluation with the normal and reasonably foreseeable use, exposure data, MoS calculation, toxicological profile of each substance. |
| 11. Undesirable effects statement |
| 12. Other tests if any |
| 13. The diploma of the safety assessor |
| 14. A description of the method of manufacturing |
| 15. A statement on compliance with good manufacturing practice |
| 16. Where justified by the nature or the effect of the cosmetic product, proof of the effect claimed for the cosmetic product |
| 17. Data on any animal testing on the finished product and on each raw material |
| 18. The labels |
| 19. PIFs must be in English |
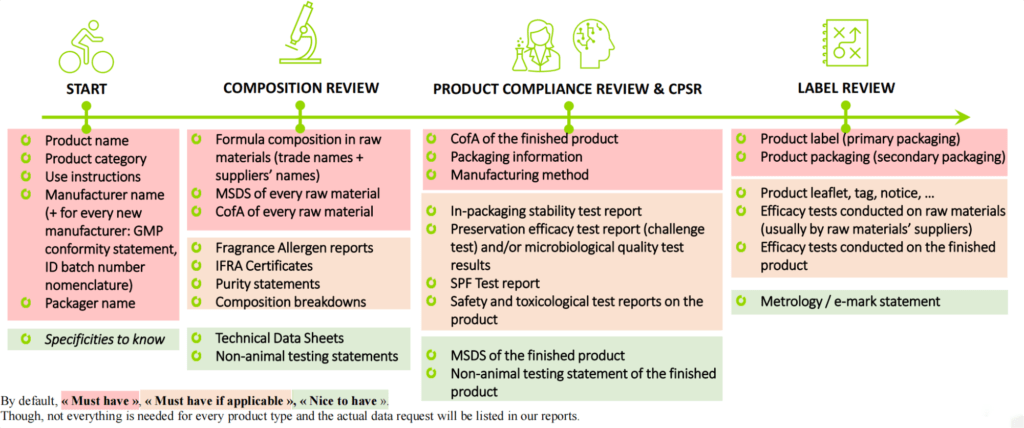
Step 2: Identify Regulatory Requirements
Research the specific regulatory requirements for cosmetic products in the regions where you plan to sell them. Different countries may have varying regulations, so ensure your PIF meets all the necessary standards.
Step 3: Create a Cosmetic Product Information File (PIF) Template
Design a standardized template for your PIFs. This template should include sections for product description, ingredients, manufacturing process, safety assessments, and more. Having a consistent format makes it easier to organize information across different products, check out the standardized Cosmetic Product Information File(PIF) template.
Step 4: Describe the Product
Provide a detailed description of the cosmetic product. Include its name, intended use, target audience, and any unique selling points.
Step 5: Formulation Details
List all the ingredients used in the product’s formulation. Include their INCI (International Nomenclature of Cosmetic Ingredients) names, concentrations, and functions in the formula.
Step 6: Manufacturing Process
Explain the process of manufacturing the product. Detail each step, from ingredient mixing to packaging, to ensure transparency and accuracy.
Step 7: Packaging Information
Describe the packaging materials and design. Include information about the container’s size, shape, and any special features that contribute to the product’s safety and efficacy.
Step 8: Safety Assessments
Provide a thorough safety assessment for the product. This includes information on potential risks, toxicological data, and any studies conducted to ensure consumer safety.
Step 9: Exposure and Use Information
Explain how consumers should use the product. Include instructions, warnings, and precautions to ensure proper usage and minimize risks.
Step 10: Test Results and Certifications
If applicable, include results from efficacy and stability tests, as well as any relevant certifications your product has obtained.
Step 11: Regulatory Compliance
Detail how your product complies with regulatory guidelines, including labeling requirements and any claims made on the packaging.
Step 12: Continuous Monitoring
Outline how you will continuously monitor the product’s safety and effectiveness. This includes plans for post-market surveillance and handling customer feedback.
Step 13: Updates and Revisions Of Cosmetic Product Information File (PIF)
Explain how you will update the PIF as new information becomes available. This may include changes to the formulation, manufacturing process, or safety data.
Step 14: Documentation and Organization
Ensure that all information in the PIF is well-organized and properly documented. Clear references and citations should be provided for safety assessments and test results.
Step 15: Review and Approval Of Your Cosmetic Product Information File
Before finalizing the PIF, have it reviewed by relevant experts, such as toxicologists and regulatory specialists, to ensure accuracy and compliance.
Developing a comprehensive and accurate PIF is crucial for the safety and success of your cosmetic product. By following these steps, you can create PIFs that provide regulators and consumers with the necessary information to make informed decisions.
Remember, the PIF should always be up-to-date and reflect the most current information about your product.
To simplify the process, it may be helpful to seek guidance from regulatory affairs experts like SGS or Intertek, as there are many steps involved.
UK And European Registration Process Requires 5 Important Steps For Cosmetic Products:
Step 1: Formula Review
The first step is a Formula Review:
- Regulatory and Safety Compliance of the Ingredients (because some ingredients are restricted/prohibited)
- Establishment of the INCI List
- Confirmation of the mandatory lab tests
This step is a prerequisite for the preparation of the CPSR (Cosmetic Product Safety Report) and the verification of the product label.
The formula review is a meticulous review of your cosmetics formula to ensure that all ingredients are safe for their intended use and comply with the European cosmetics regulation:
- The composition is entirely rebuilt from its raw materials and the formula is expressed in trade names. The raw materials documentation is reviewed to verify its regulatory compliance, and the impurities profile is highlighted.
- Preliminary calculations and investigations are conducted to ensure that each ingredient and impurity is safe, given the cosmetic product, its intended use and the targeted population.
Step 2: Cosmetic Product Safety Report (CPSR)
The CPSR (Cosmetic Product Safety Report) A&B (signed by a Toxicologist) is a long and complex file of the EU cosmetic regulation.
The CPSR is a comprehensive document produced by a qualified toxicologist. This safety report intends to support and confirm the safe use of the cosmetic product, taking into consideration all pieces of information available, such as toxicological profiles of each ingredient and impurity, test results, certificates, declarations, raw materials documentation, etc. Introducing a cosmetic product to the EU market without a high-quality CPSR is a serious offensive that typically leads to withdrawal from the market, significant financial penalties and serious damage to the cosmetic brand’s reputation.
The CPSR takes the form of two distinct sections, Part A and Part B.
Part A: Cosmetic Product Safety Information
This part of the report contains all data needed for the evaluation of the cosmetic product.
Part B: Cosmetic Product Safety Assessment
This part of the report includes an evaluation of the cosmetic product’s safety and conclusions. The Part B is essential, as it certifies the effectiveness and safety of a product before being placed on the EU market.
Step 3: Product Information File (PIF) and CPNP Notification
A PIF (Cosmetic Product Information File) is a complete cosmetic regulatory file that contains:
- Formula
- CPSR A&B
- Labels
A Product Information File is a large and highly-structured dossier containing every piece of information related to a given cosmetic product. Some of the data come from product manufacturers, other data from independent laboratories, and yet other data comes from a duly-qualified safety assessor.
Once the PIF has been completed, the cosmetic product can be notified electronically in the European Commission via the CPNP (Cosmetic Product Notification Portal) and a unique CPNP number is delivered. This number can be requested from the importer and the Responsible Person at any time, notably by customs officers.
In other words, a notification is to announce to Europe that you will sell a product in Europe.
Technically, cosmetic products are not registered in the EU; they are notified. Unlike other countries and regions that require pre-approval of the products by the competent authorities, the EU requires notification before the product can be launched in its market. This means that verification by the competent authorities is performed after the product is launched in the EU market. For this reason, selecting a qualified and competent Responsible Person is especially important.
Step 4: Labels and Claims Review
The EU cosmetic labelling rules may seem very confusing and although it falls under the responsibility of the distributors (according to Article 6 of the EU cosmetics regulation), the design and edition of labels and packaging are and remain a costly exercise for cosmetic brand owners.
Some elements will indeed have to be translated into all the official languages of the countries where the products are sold, and Distributors have to ensure this info is properly translated. Labels only in English are indeed not always sufficient. The labels (or certain parts of the labels) indeed must be translated in the country’s language, but it really depends on each EU country. Welcome to EU, one of the largest market for cosmetic products in the world… and their 27 countries with their 24 different languages!
The distributors are of course fully entitled to refuse products that would not comply with these requirements.
A clear label is important to help consumers for their purchases and to protect their health, all important information always has to be easily accessible, readable and understandable by the consumer at the time of purchase. The rule is actually quite simple: the end-consumer has to understand precisely what he is buying when he is checking the product.
A Label Review is a complete compliance of packaging and labels and the experts will check everything on the packaging and labels: claims, PAO (Period After Opening), warnings, INCI list, … .
In summary, making a Label Review in Europe (complete review and revision of labels and claims that take into account all European regulations) and a consultancy on specific claims or topics that the brand wants to promote as marketing text (by a recognized and renown company ) is the best idea for your business.
Step 5: Legal Representation
The Legal Representation for cosmetics in Europe is called the Responsible Person. The Responsible Person comes with many responsibilities and the Responsible Person will be held responsible in the event of a non-compliance problem.
A simple and easy way to explain the Responsible Person is:
- Who do European countries have to call if they want to control your cosmetic product?
- Who should a consumer call if they have an unusual reaction to your cosmetic product?
A Responsible Person is designated in CPNP for each notified product. Among other important functions, this Responsible Person will ensure the compliance of the cosmetic products on an ongoing basis and will update the PIF when necessary.
Conclusion: Empowering Consumers Through PIF Knowledge
PIFs might seem like a behind-the-scenes aspect of the cosmetic industry, but they are instrumental in upholding consumer safety. By understanding PIFs, consumers can take charge of their choices and prioritize products that adhere to rigorous quality standards.
FAQs (Frequently Asked Questions)
1. What is the primary purpose of a Cosmetic Product Information File (PIF)?
A Cosmetic Product Information File (PIF) serves as a comprehensive dossier containing essential information about a cosmetic product, ensuring its safety and compliance with regulations.
2. Can you explain the difference between a PIF and product labeling?
While product labeling provides basic information, PIFs offer in-depth details about a product’s formulation, safety assessments, and more.
3. Who is responsible for creating and maintaining PIFs?
Manufacturers are responsible for creating and regularly updating PIFs to reflect accurate and current product information. But the cost will burden to the clients
4. How often should a PIF be updated?
PIFs should be updated whenever there are changes to a product’s formulation, safety data, or other relevant information.
5. Are PIFs applicable to all cosmetic products?
Yes, PIFs are required for all cosmetic products that are intended to be placed on the market for consumer use.